Abstract
Introduction
Chromosomal instability (CIN) is a driving force of myelomagenesis: the continuous modification of plasma cell (PC) genomes favours the acquisition of progressively more DNA alterations, clonal evolution and heterogeneity, thereby promoting tumour development. Similarly, the pliancy of Multiple Myeloma (MM) plasma cells differentiation status acts as adaptive strategy to exogenous stress (e.g. in response to therapy). However, the genomic background that supports any diverse plasma cell differentiation phenotype has not yet been inferred.
Aim
To correlate the genomic background with the phenotypic plasticity of MM clone(s) at diagnosis, in order to stratify patients (pts) according to both the level of CIN and their PC differentiation stages, and ultimately to evaluate the impact of this stratification on the disease outcome.
Patients and Methods
A total of 64 newly diagnosed pts were included in the present study. Nearly all pts (54/64) received a PI-based treatment as a front-line therapy. In each patient, both the BM CD138+/CD38+ PC and CD19+ B cell compartments were characterized by 6-color multiparametric flow citometry analysis, combining CD138-PE, CD38-PE-Cy7, CD20-APC, CD19-APC-Cy7, CD27-FITC, CD45-FITC, CD28-APC, CD44-FITC, CD54-APC, CD81-PerCP-Cy5.5, CD56-APC and SHH-PE, as a functional marker of Hedgehog pathway activation (Miltenyi Biotech). Whole copy number abnormalities (CNAs) characterization of CD138+ purified BM PCs was carried out by SNP array hybridization with Cytoscan HD array (Affymetrix).
Results
According to the detected CIN, as described both by total CNAs and portion of genome changed (GC), three major subgroups were identified: the first one, including 21 pts with high CIN (medium tot. CNAs = 550, % GC ≥ 25%); the second one including 25 pts with a intermediate CIN (medium tot. CNAs = 315, % GC = 10-25%) and the third one including 18 pts with low CIN (medium tot. CNAs = 105, % GC ≤ 10%). As expected, in pts with high CIN, more than a quarter of unstable genome was due to the hyperdiploidy; however, they were also characterized by a higher prevalence of high-risk features. Indeed, 1p deletion (FAF1), 16q deletion (WWOX, FANCA) and 17p deletion (TP53) were the most recurrent abnormalities, and almost exclusively associated to high CIN pts (p<.05). A concordance analysis between patients' subgroup reveals that the presence of these three deletions positively correlates with an overall deregulation of other tumour suppressor genes, distributed along the genome, such as RB1, LATS2, TNFAIP3, ST13 and NF1 (p<.05). This particular genomic make-up was also associated to a marked oncogenes amplification on MYC, MYCN, IRF4, EGFR, CCND1 and AKT2 genes (p<.05), which probably reflects their activation.
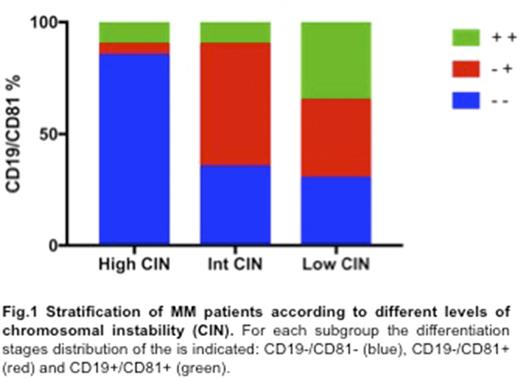
A detailed immunophenotypic analysis of the three subgroups of pts showed that high CIN background mainly characterized mature PCs (17/21 = 81%) (Fig. 1), as described by: a) a significant deregulation of both CD19 and CD81 markers; b) a higher expression of CD28 and CD44, which usually characterized advanced disease stages; c) a reduced expression of CD20, CD27 and CD45, commonly associated to preceding PC differentiation stages (p<.05). Accordingly, considering the MM clone(s) as a mixture of different B-cell lineage populations, the high CIN pts advanced differentiation status was also confirmed by a reduced Hedgehog pathway activation in the CD19+ B cells compartment (median SHH expression CD19-/CD81- vs. CD19+/CD81+: 11,3% vs. 98,1%; p<.05), as well as by a declining of CD138-/low/CD19+/CD20-/CD38high/CD27high plasmablast population, which probably highlighted a more quiescent immature reservoir pool.
Finally, the presence of more mature PCs with high CIN characterizes pts carrying baseline clinical features associated to bad prognosis (e.g. PET lesions, k/l ratio, ISS III, β2-microglobulin; p<.05). In addition, these pts tend to obtain high quality response rates (≥CR) to PI induction therapy.
Conclusion
A high level of genomic complexity correlates with an advanced PCs differentiation stages in newly diagnosed MM patients, and this is lastly associated with a prevalence of poor prognosis features. Chromosomal instability, together with cellular phenotypic pliancy, represents an important, yet poorly defined, mechanism by which MM clone(s) accelerate their own evolution and survival.
Acknowledgments: AIRC, AIL, Fondazione Berlucchi.
Martinelli: Ariad/Incyte: Consultancy; Johnson&Johnson: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal